Transdermal Bioavailability: Formulating Cannabis Topicals for Systemic vs. Localized Effects
Introduction
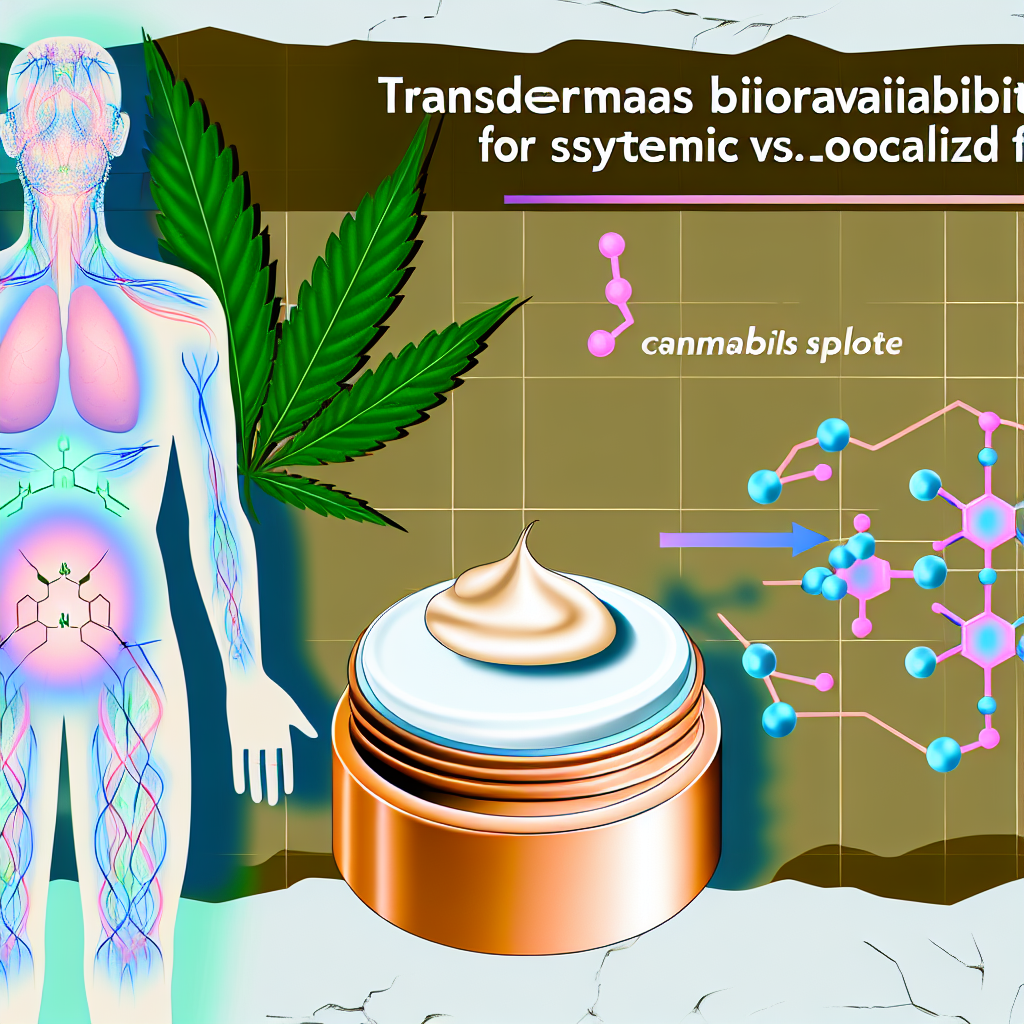
As the cannabis industry continues to evolve, topical applications of cannabinoids are gaining traction in both medical and recreational markets. From creams and balms to patches and gels, these products are sought for targeted relief. However, a critical distinction exists between whether a topical is designed for localized or systemic effects—primarily determined by its ability to breach the skin barrier and deliver cannabinoids into the bloodstream through transdermal absorption.
Transdermal bioavailability refers to how efficiently a drug or cannabinoid is absorbed through the skin and enters the systemic circulation. Traditional topicals like CBD lotions or THC balms typically provide localized relief without bloodstream penetration. In contrast, transdermal cannabis systems—such as patches and high-tech gels—are engineered to bypass skin defenses and release cannabinoids into the body, creating full-body therapeutic effects.
This formulation difference is not purely functional—it is also rooted in biology. Human skin, especially the outermost layer known as the stratum corneum, forms a formidable barrier. Lipophilic and tightly packed, it resists penetration by most molecules. Although cannabinoids such as THC and CBD are lipophilic, their high molecular weights further complicate absorption. Successful transdermal formulations must overcome these obstacles through the inclusion of penetration enhancers (e.g., ethanol, oleic acid), nano-delivery systems like nanoemulsions or liposomes, and specific pH optimization to facilitate effective delivery.
For product developers, healthcare professionals, and informed consumers, understanding the science of transdermal delivery is critical. The differences in formulation and bioavailability affect not just efficacy and onset time but also the therapeutic goal—whether the aim is localized relief or systemic support for broader symptoms such as anxiety or neuropathic pain.
This article unpacks the science behind transdermal bioavailability, contrasts systemic and localized delivery, and explores medical research supporting this growing segment in the cannabis product market.
Features: Professional and Medical Studies Supporting Transdermal Cannabis Use
Numerous medical and pharmaceutical studies support the efficacy and innovation driving transdermal cannabis delivery systems. These studies highlight the complexity of cannabinoid absorption and the strategies employed to address biological challenges.
One notable study in the European Journal of Pharmaceutical Sciences explored transdermal delivery of THC and CBD using chemical permeation enhancers. Cannabinoids inherently challenge the skin barrier due to their high molecular weights and lipophilic nature. The study showed that using ethanol and oleic acid as permeation enhancers significantly improved cannabinoid transport through the skin, suggesting promising formulations for systemic relief.
Further evidence comes from a 2017 study published in Pain, a leading medical journal, which examined the use of transdermal CBD to treat arthritis in a rat model. Results showed marked decreases in inflammation and pain behaviors, with no observable side effects frequently associated with oral cannabinoid administration. This supports the theory that full systemic saturation is not always required—localized transdermal routes can still yield therapeutic effects from plasma-level exposure.
Additionally, insights from the Journal of Controlled Release emphasized the role of nanotechnology in enhancing transdermal delivery. Using nanoemulsions and liposomal carriers to encapsulate cannabinoids improves their stability, increases their ability to penetrate skin layers, and prolongs drug release profiles—offering greater dosing consistency and improved bioavailability.
Commercially, products like the well-known transdermal patches from companies such as Mary’s Medicinals demonstrate real-world application of this science. These patches utilize carriers like propylene glycol, lecithin, and alcohol-water blends to deliver measurable cannabinoids beyond the skin. Case studies report significant benefits including reductions in chronic pain, menstrual discomfort, and generalized anxiety.
In terms of regulatory alignment, the U.S. Food and Drug Administration (FDA) has long-standing guidance for other transdermal drugs such as estrogen or nicotine patches. This precedent is crucial for aspiring cannabis pharmaceutical brands seeking clinical acceptance. Developers are now aligning cannabinoid patch technologies with FDA standards to position themselves for future regulatory approval and greater medical legitimacy. You can view the FDA’s full guidance here.
Ultimately, successful cannabis transdermals require multidisciplinary expertise. Combining pharmacokinetics, formulation chemistry, and topical delivery techniques ensures cannabinoids are delivered effectively and safely—whether the desired effect is systemic or localized.
Conclusion
The formulation of cannabis topicals for either systemic or localized effects is a dynamic interaction of science, biology, and regulatory strategy. As innovation accelerates within this space, understanding the fundamental mechanisms behind transdermal bioavailability becomes more than a technical requirement—it becomes a competitive advantage.
With increased access to clinical evidence and formulation technologies, developers and healthcare professionals can now create more effective, consistent, and targeted cannabinoid therapies. For consumers, this means better outcomes, clearer dosing expectations, and a safer experience.
As the market for cannabis-infused topicals continues to mature, embracing a deeper knowledge of skin absorption science and product engineering may determine which products truly deliver on their promises—and which simply stay on the surface.
References
– FDA Guidance for Industry: Transdermal Drug Delivery Systems
For more cannabis science education and formulation strategies, visit Bluntys.com.
Concise Summary
Transdermal cannabis topicals differ from traditional lotions or balms because they deliver cannabinoids into the bloodstream, offering systemic therapeutic effects. Achieving this requires overcoming the skin’s natural barrier with advanced formulations using permeation enhancers and nanotechnology. Supported by clinical studies and pharmaceutical techniques, transdermal systems provide predictable dosing and improved absorption. Professionals must understand formulation science to design effective products for pain, anxiety, inflammation, and beyond. With evolving research and FDA-aligned strategies, transdermal cannabis applications are paving the way for a more consistent and medically viable future.